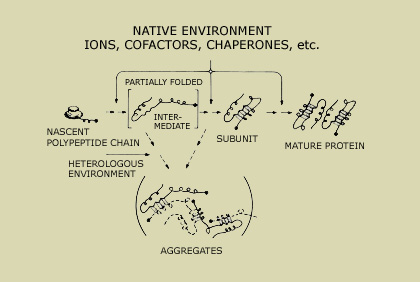
Model pathway for a newly synthesized polypeptide chain emerging from the ribosome and folding to its native state, as well as an off-pathway inactive aggregated state. (Image courtesy of Professor Jonathan King.)
Course Highlights
The research paper and oral presentation are essential components of the course curriculum. Students must select their area of research from an extensive list of current topics in protein folding, misfolding, and their physiological roles. Topics can be found in the
Projects section of this course.
Course Description
This course focuses on the mechanisms by which the amino acid sequence of polypeptide chains (proteins), determine their three-dimensional conformation. Topics in this course include sequence determinants of secondary structure, the folding of newly synthesized polypeptide chains within cells, folding intermediates aggregation and competing off-pathway reactions, and the unfolding and refolding of proteins in vitro. Additional topics covered are the role of helper proteins such as chaperonins and isomerases, protein recovery problems in the biotechnology industry, and diseases found associated with protein folding defects.
*Some translations represent previous versions of courses.